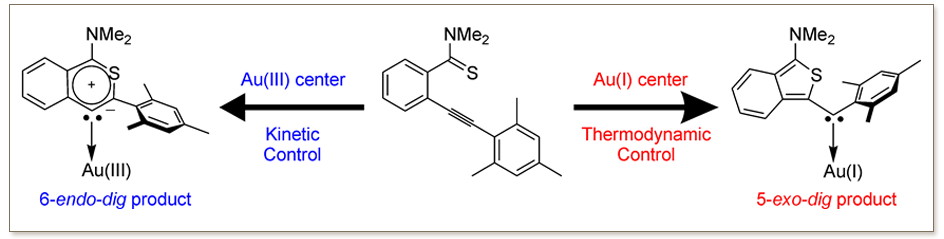
We demonstrate that the experimentally observed switch in selectivity from 5-exo-dig to 6-endo-dig cyclization of an alkynyl substrate, promoted by Au I and AuIII complexes, is connected to a switch from thermodynamic to kinetic reaction control. The AuIII center pushes alkyne coordination toward a single Au-C(alkyne) σ-bond, conferring carbocationic character (and reactivity) to the distal alkyne C atom.
10.1021/cs5001575