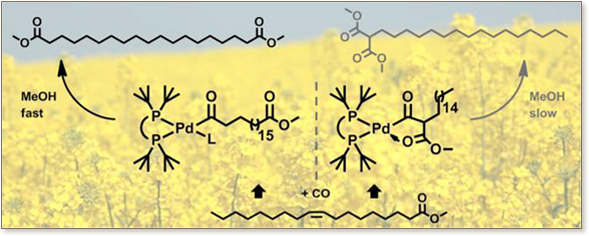
The weakly coordinated triflate complex [(P̂P)Pd(OTf)] +(OTf)- (1) (P̂P = 1,3-bis(di-tert- butylphosphino)propane) is a suitable reactive precursor for mechanistic studies of the isomerizing alkoxcarbonylation of methyl oleate. Addition of CH 3OH or CD3OD to 1 forms the hydride species [(P ̂P)PdH(CH3OH)]+(OTf)- (2-CH3OH) or the deuteride [(P̂P)PdD(CD 3OD)]+(OTf)- (2D-CD3OD), respectively. Further reaction with pyridine cleanly affords the stable and isolable hydride [(P̂P)PdH(pyridine)]+(OTf) - (2-pyr). This complex yields the hydride fragment free of methanol by abstraction of pyridine with BF3OEt2, and thus provides an entry to mechanistic observations including intermediates reactive toward methanol. Exposure of methyl oleate (100 equiv) to 2D-CD 3OD resulted in rapid isomerization to the thermodynamic isomer distribution, 94.3% of internal olefins, 5.5% of α,β-unsaturated ester and <0.2% of terminal olefin. Reaction of 2-pyr/BF3OEt 2 with a stoichiometric amount of 1-13C-labeled 1-octene at -80 °C yields a 50:50 mixture of the linear alkyls [(P ̂P)Pd13CH2(CH2) 6CH3]+ and [(P̂P)PdCH 2(CH2)6 13CH3] + (4a and 4b). Further reaction with 13CO yields the linear acyls [(P̂P)Pd13C(=O)12/13CH 2(CH2)6 12/13CH3(L)] + (5-L; L = solvent or 13CO). Reaction of 2-pyr/BF 3·OEt2 with a stoichiometric amount of methyl oleate at -80 °C also resulted in fast isomerization to form a linear alkyl species [(P̂P)PdCH2(CH2) 16C(=O)OCH3]+ (6) and a branched alkyl stabilized by coordination of the ester carbonyl group as a four membered chelate [(P̂P)PdCH{(CH2)15CH 3}C(=O)OCH3]+ (7). Addition of carbon monoxide (2.5 equiv) at -80 °C resulted in insertion to form the linear acyl carbonyl [(P̂P)PdC(=O)(CH2)17C(=O)OCH 3(CO)]+ (8-CO) and the five-membered chelate [(P ̂P)PdC(=O)CH{(CH2)15CH3}C(=O) OCH3]+ (9). Exposure of 8-CO and 9 to 13CO at -50 °C results in gradual incorporation of the 13C label. Reversibility of 7 + CO ⇄ 9 is also evidenced by ΔG = -2.9 kcal mol-1 and ΔG† = 12.5 kcal mol-1 from DFT studies. Addition of methanol at -80 °C results in methanolysis of 8-L (L = solvent) to form the linear diester, 1,19-dimethylnonadecandioate, whereas 9 does not react and no branched diester is observed. DFT yields a barrier for methanolysis of ΔG† = 29.7 kcal mol -1 for the linear (8) vs ΔG† = 37.7 kcal mol-1 for the branched species (9).