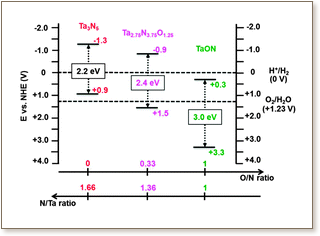
 Finding an ideal photocatalyst for achieving efficient overall water splitting still remains a great challenge. By applying accurate first-principles quantum calculations based on DFT with the screened non-local hybrid HSE06 functional, we bring rational insights at the atomic level into the influence of non-stoichiometric compositions on essential properties of tantalum (oxy)nitride compounds as visible-light-responsive photocatalysts for water splitting. Indeed, recent experiments show that such non-stoichiometry is inherent to the nitridation methods of tantalum oxide with unavoidable oxygen impurities. We considered here O-enriched Ta3N5 and N-enriched TaON materials. Although their structural parameters are found to be very similar to those of pure compounds and in good agreement with available experimental studies, their photocatalytic features for visible-light-driven overall water splitting reactions show different behaviors. Further partial nitration of TaON leads to a narrowed band gap, but partially oxidizing Ta3N5 causes only subtle changes in the gap. The main influence, however, is on the band edge positions relative to water redox potentials. The pure Ta3N5 is predicted to be a good candidate only for H+ reduction and H2 evolution, while the pure TaON is predicted to be a good candidate for water oxidation and O2 evolution. Non-stoichiometry has here a positive influence, since partially oxidized tantalum nitride, Ta(3x)N(5-5x)O5x (for x ≥ 0.16) i.e. with a composition in between TaON and Ta3N5, reveals suitable band edge positions that correctly bracket the water redox potentials for visible-light-driven overall water splitting reactions. Among the various explored Ta(3x)N(5-5x)O5x structures, a strong stabilization is obtained for the configuration displaying a strong interaction between the O-impurities and the created Ta-vacancies. In the lowest-energy structure, each created Ta-vacancy is surrounded by five O-impurity species substituting the five N sites characterizing one octahedral environment.
Finding an ideal photocatalyst for achieving efficient overall water splitting still remains a great challenge. By applying accurate first-principles quantum calculations based on DFT with the screened non-local hybrid HSE06 functional, we bring rational insights at the atomic level into the influence of non-stoichiometric compositions on essential properties of tantalum (oxy)nitride compounds as visible-light-responsive photocatalysts for water splitting. Indeed, recent experiments show that such non-stoichiometry is inherent to the nitridation methods of tantalum oxide with unavoidable oxygen impurities. We considered here O-enriched Ta3N5 and N-enriched TaON materials. Although their structural parameters are found to be very similar to those of pure compounds and in good agreement with available experimental studies, their photocatalytic features for visible-light-driven overall water splitting reactions show different behaviors. Further partial nitration of TaON leads to a narrowed band gap, but partially oxidizing Ta3N5 causes only subtle changes in the gap. The main influence, however, is on the band edge positions relative to water redox potentials. The pure Ta3N5 is predicted to be a good candidate only for H+ reduction and H2 evolution, while the pure TaON is predicted to be a good candidate for water oxidation and O2 evolution. Non-stoichiometry has here a positive influence, since partially oxidized tantalum nitride, Ta(3x)N(5-5x)O5x (for x ≥ 0.16) i.e. with a composition in between TaON and Ta3N5, reveals suitable band edge positions that correctly bracket the water redox potentials for visible-light-driven overall water splitting reactions. Among the various explored Ta(3x)N(5-5x)O5x structures, a strong stabilization is obtained for the configuration displaying a strong interaction between the O-impurities and the created Ta-vacancies. In the lowest-energy structure, each created Ta-vacancy is surrounded by five O-impurity species substituting the five N sites characterizing one octahedral environment.